Alkaline cell hydrogen production is a relatively mature electrolytic hydrogen production technology. Alkaline cell is safe and reliable, with a life span of 15 years, and has been widely used commercially. The working efficiency of alkaline cell is generally 42% ~ 78%. In the past few years, alkaline electrolytic cells have made progress in two main aspects. On the one hand, the improved cell efficiency has been improved and the operating costs associated with electricity consumption have been reduced. On the other hand, the operating current density increases and the investment cost decreases.
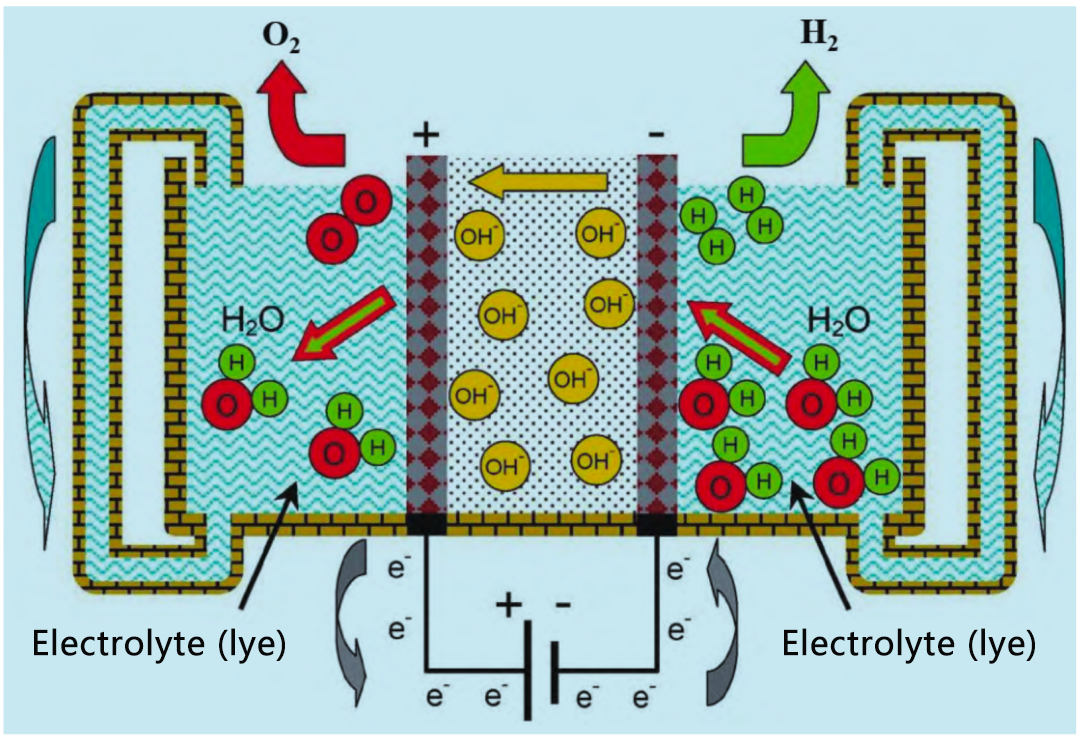
The working principle of alkaline electrolyzer is shown in the figure. The battery consists of two electrodes separated by an air-tight diaphragm. Battery assembly is immersed in a high concentration of alkaline liquid electrolyte KOH(20% to 30%) to maximize ionic conductivity. NaOH and NaCl solutions can also be used as electrolytes, but they are not commonly used. The main disadvantage of electrolytes is that they are corrosive. The cell operates at a temperature of 65 °C to 100°C. The cathode of the cell produces hydrogen, and the resulting OH – flows through the diaphragm to the anode, where it recombines to produce oxygen.
Advanced alkaline electrolytic cells are suitable for large-scale hydrogen production. Alkaline electrolytic cells made by some manufacturers have very high hydrogen production capacity at (500 ~ 760Nm3/h), with corresponding power consumption of 2150 ~ 3534kW. In practice, to prevent the creation of flammable gas mixtures, the hydrogen yield is limited to 25% to 100% of the rated range, the maximum allowable current density is about 0.4A/cm2, the operating temperature is 5 to 100°C, and the maximum electrolytic pressure is close to 2.5 to 3.0 MPa. When the electrolytic pressure is too high, the investment cost increases and the formation risk of harmful gas mixture increases significantly. Without any auxiliary purification device, the purity of hydrogen produced by alkaline cell electrolysis can reach 99%. Alkaline electrolytic cell electrolytic water must be pure, in order to protect the electrode and safe operation, water conductivity is less than 5S/cm.
Post time: Feb-02-2023