In the field of advanced energy storage, flow batteries have gradually emerged as a scalable and long-duration solution, particularly for stationary applications such as grid balancing, renewable energy integration, and industrial backup systems. Among the core materials that determine the performance and longevity of these systems, graphite felt stands out as a crucial component—especially within the electrode architecture.
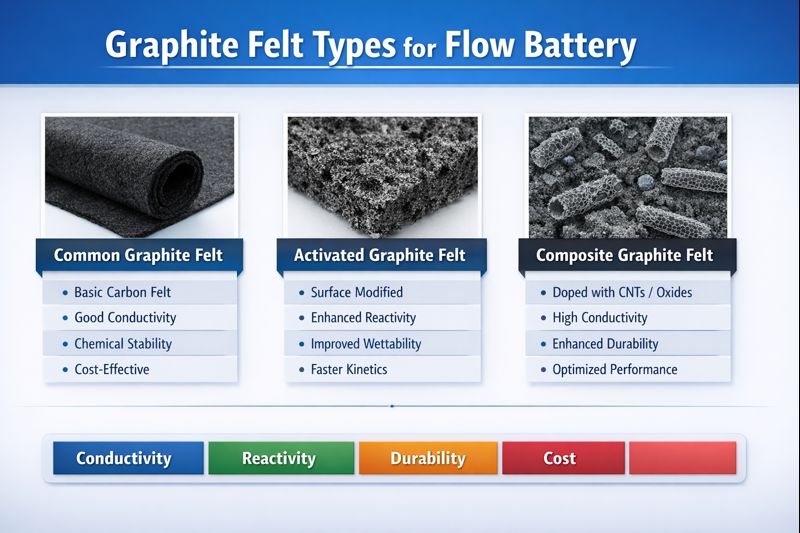
Graphite felt is a porous, carbon-based material with high conductivity, chemical resistance, and thermal stability. These properties make it exceptionally well-suited for flow battery systems, where liquid electrolytes continuously pass through electrochemical cells during charging and discharging cycles. Unlike traditional batteries where the electrodes are compact and fixed, flow batteries rely on constant fluid movement across electrode surfaces. Graphite felt, due to its fibrous network and large surface area, provides an efficient medium for electron transfer and redox reactions.
In vanadium redox flow batteries (VRFBs), which are among the most commercially mature types, graphite felt is commonly used for both positive and negative electrodes. The high surface area promotes effective contact with vanadium ions in the electrolyte, while the material’s stability under strongly acidic environments ensures durability over thousands of cycles. Moreover, its flexible structure allows engineers to shape or compress the felt to optimize contact pressure, reduce internal resistance, and improve overall current efficiency.
The manufacturing of graphite felt typically involves the carbonization of synthetic fibers, such as PAN (polyacrylonitrile), under controlled atmospheres, followed by optional thermal or chemical activation treatments. These post-treatments further enhance the electrochemical activity of the surface, creating more catalytic sites for redox reactions. Advanced variants of graphite felt may also be doped or coated with metal oxides or other functional layers to improve selectivity, reduce polarization losses, and accelerate reaction kinetics.
One notable advantage of graphite felt over metallic or rigid carbon-based electrodes lies in its three-dimensional microstructure. The interconnected fiber network not only facilitates uniform electrolyte distribution but also tolerates minor flow disturbances or pressure fluctuations, which are common in large-scale energy storage systems. This helps maintain consistent electrochemical performance even under dynamic load conditions.
In practical systems, graphite felt is not a plug-and-play component. Its performance is highly dependent on cell design, compression ratio, electrolyte composition, and operating temperature. Engineers must carefully balance porosity, conductivity, and compressibility when selecting the right felt material. Too low a density may lead to increased ohmic losses, while overly dense felts can restrict fluid movement and reduce ion transport rates.
Ongoing research is exploring ways to push the boundaries of graphite felt performance. One direction involves modifying the fiber surfaces to introduce functional groups that selectively promote specific redox couples. Another focus is on hybrid felts that combine graphite with other conductive materials like carbon nanotubes or graphene to improve mechanical strength and surface reactivity without sacrificing conductivity.
As flow battery technology continues to evolve and find broader adoption, the role of graphite felt is likely to become more critical. From residential energy storage to megawatt-scale grid systems, the need for robust, low-maintenance, and high-performance electrode materials remains constant. Graphite felt, with its unique combination of structure and functionality, remains a cornerstone of this development.
Post time: Dec-29-2025