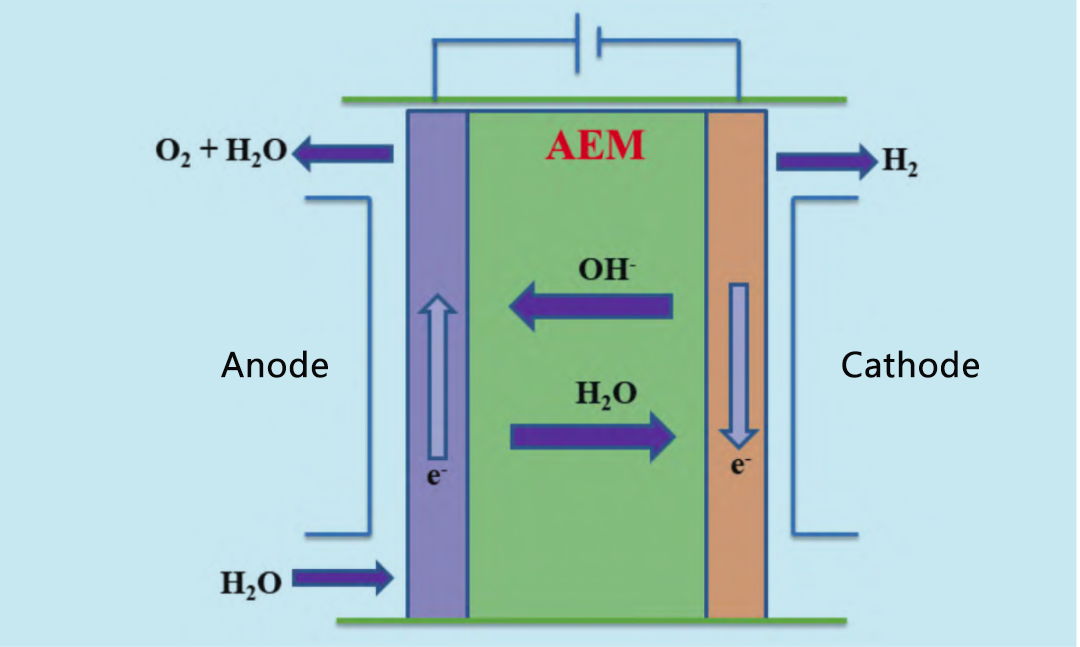
AEM is to some extent a hybrid of PEM and traditional diaphragm based lye electrolysis. The principle of AEM electrolytic cell is shown in Figure 3. At the cathode, water is reduced to produce hydrogen and OH -. OH — flows through the diaphragm to the anode, where it recombines to produce oxygen.
Li et al. [1-2] studied highly quaternized polystyrene and polyphenylene AEM high-performance water electrolyzer, and the results showed that the current density was 2.7A/cm2 at 85°C at a voltage of 1.8V. When using NiFe and PtRu/C as catalysts for hydrogen production, the current density decreased significantly to 906mA/cm2. Chen et al. [5] studied the application of high-efficiency non-noble metal electrolytic catalyst in alkaline polymer film electrolyzer. NiMo oxides were reduced by H2/NH3, NH3, H2 and N2 gases at different temperatures to synthesize electrolytic hydrogen production catalysts. The results show that the NiMo-NH3/H2 catalyst with H2/NH3 reduction has the best performance, with current density up to 1.0A/cm2 and energy conversion efficiency of 75% at 1.57V and 80°C. Evonik Industries, based on its existing gas separation membrane technology, has developed a patented polymer material for use in AEM electrolytic cells and is currently expanding membrane production on a pilot line. The next step is to verify the reliability of the system and improve battery specifications, while scaling up production.
At present, the main challenges facing AEM electrolytic cells are the lack of high conductivity and alkaline resistance of AEM, and the precious metal electrocatalyst increases the cost of manufacturing electrolytic devices. At the same time, CO2 entering the cell film will reduce the film resistance and electrode resistance, thus reducing the electrolytic performance. The future development direction of AEM electrolyzer is as follows: 1. Develop AEM with high conductivity, ion selectivity and long-term alkaline stability. 2. Overcome the problem of high cost of precious metal catalyst, develop catalyst without precious metal and high performance. 3. Currently, the target cost of AEM electrolyzer is $20 /m2, which needs to be reduced through cheap raw materials and reduced synthesis steps, so as to reduce the overall cost of AEM electrolyzer. 4. Reduce CO2 content in electrolytic cell and improve electrolytic performance.
[1] Liu L,Kohl P A. Anion conducting multiblock copolymers with different tethered cations[J].Journal of Polymer Science Part A: Polymer Chemistry, 2018, 56(13): 1395 — 1403.
[2] Li D, Park E J, Zhu W,et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers[J]. Nature Energy, 2020, 5: 378 — 385.
Post time: Feb-02-2023