Progress and economic analysis of hydrogen production by electrolysis of solid oxides
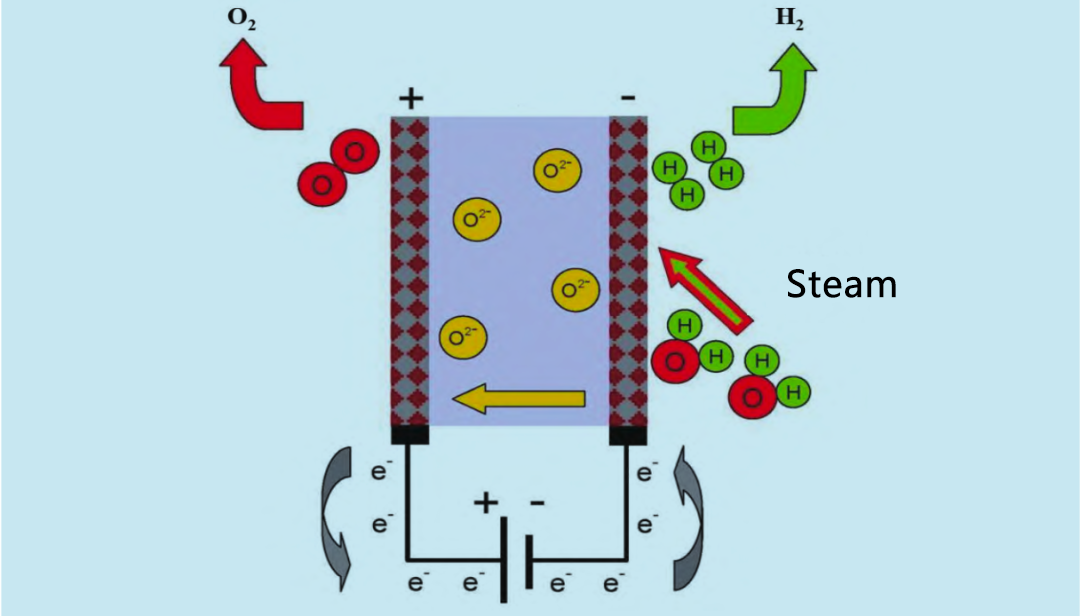
Solid oxide electrolyzer (SOE) uses high-temperature water vapor (600 ~ 900°C) for electrolysis, which is more efficient than alkaline electrolyzer and PEM electrolyzer. In the 1960s, the United States and Germany began to conduct research on high-temperature water vapor SOE. The working principle of SOE electrolyzer is shown in Figure 4. Recycled hydrogen and water vapor enter the reaction system from the anode. The water vapor is electrolyzed into hydrogen at the cathode. The O2 produced by the cathode moves through the solid electrolyte to the anode, where it recombines to form oxygen and release electrons.
Unlike alkaline and proton exchange membrane electrolytic cells, the SOE electrode reacts with water vapor contact and faces the challenge of maximizing the interface area between the electrode and water vapor contact. Therefore, the SOE electrode generally has a porous structure. The purpose of water vapor electrolysis is to reduce the energy intensity and reduce the operating cost of conventional liquid water electrolysis. In fact, although the total energy requirement of the water decomposition reaction increases slightly with increasing temperature, the electrical energy requirement decreases significantly. As the electrolytic temperature increases, part of the energy required is supplied as heat. The SOE is capable of producing hydrogen in the presence of a high-temperature heat source. Since high-temperature gas-cooled nuclear reactors can be heated to 950°C, nuclear energy can be used as an energy source for the SOE. At the same time, the research shows that the renewable energy such as geothermal energy also has the potential as the heat source of steam electrolysis. Operating at high temperature can reduce battery voltage and increase reaction rate, but it also faces the challenge of material thermal stability and sealing. In addition, the gas produced by the cathode is a hydrogen mixture, which needs to be further separated and purified, increasing the cost compared with conventional liquid water electrolysis. The use of proton-conducting ceramics, such as strontium zirconate, reduces the cost of SOE. Strontium zirconate shows excellent proton conductivity at about 700°C, and is conducive to the cathode to produce high purity hydrogen, simplifying the steam electrolysis device.
Yan et al. [6] reported that zirconia ceramic tube stabilized by calcium oxide was used as SOE of supporting structure, the outer surface was coated with thin (less than 0.25mm) porous lanthanum perovskite as anode, and Ni/Y2O3 stable calcium oxide cermet as cathode. At 1000°C, 0.4A/cm2 and 39.3W input power, the hydrogen production capacity of the unit is 17.6NL/h. The disadvantage of SOE is the overvoltage resulting from high ohm losses that are common at the interconnections between cells, and the high overvoltage concentration due to the limitations of vapor diffusion transport. In recent years, planar electrolytic cells have attracted much attention [7-8]. In contrast to tubular cells, flat cells make manufacturing more compact and improve hydrogen production efficiency [6]. At present, the main obstacle to the industrial application of SOE is the long-term stability of the electrolytic cell [8], and the problems of electrode aging and deactivation may be caused.
Post time: Feb-06-2023